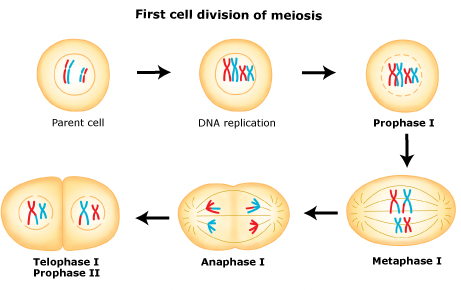
In meiosis I, homologous chromosomes pair with each other.
Oogonia undergo meiosis in their transformation to oocytes but arrest at prophase of meiosis I until the time of ovulation at puberty, age 9-10 when it resumes.
This stage of development is called the dictyate. In the dictyate (prolonged diplotene) stage actively repairs DNA damage.
Also at this stage the chromosomes can exchange genetic material in a process called chromosomal crossover. The homologous chromosomes are then pulled apart into two new separate daughter cells, each containing half the number of chromosomes as the parent cell. Thus meiosis I is referred to as a reductional division. At the end of meiosis I, sister chromatids remain attached and may differ from one another if crossing-over occurred.
A regular diploid human cell contains 46 chromosomes and is considered 2N because it contains 23 pairs of homologous chromosomes. However, after meiosis I, although the cell contains 46 chromatids, it is only considered as being N, with 23 chromosomes. This is because in Anaphase I the sister chromatids will remain together as the spindle fibers pull the pair toward the pole of the new cell.
Meiosis and Diversity in Gene Expression
Meiosis generates gamete genetic diversity in two ways:
(1) the independent orientation of homologous chromosome pairs along the metaphase plate during metaphase I and the subsequent separation of homologs during anaphase I allows a random and independent distribution of chromosomes to each daughter cell (and ultimately to gametes); and
(2) physical exchange of homologous chromosomal regions by homologous recombination during prophase I results in new combinations of DNA within chromosomes.
Chromosomal crossover (or crossing over) is the exchange of genetic material between homologous chromosomes that results in recombinant chromosomes. It is one of the final phases of genetic recombination, which occurs during prophase I of meiosis during a process called synapsis. Synapsis begins before the synaptonemal complex develops, and is not completed until near the end of prophase I. Crossover usually occurs when matching regions on matching chromosomes break and then reconnect to the other chromosome. This process begins in early stage of prophase I which is called leptotene.1
Crossing over was described, in theory, by Thomas Hunt Morgan. He relied on the discovery of the Belgian Professor Frans Alfons Janssens of the University of Leuven who described the phenomenon in 1909 and had called it "chiasmatypie". The term chiasma is linked if not identical to chromosomal crossover. Morgan immediately saw the great importance of Janssens' cytological interpretation of chiasmata to the experimental results of his research on the heredity of Drosophila. The physical basis of crossing over was first demonstrated by Harriet Creighton and Barbara McClintock in 1931.[2]

In meiosis II, the two cells produced during meiosis I divide again. During this division, sister chromatids detach from one another and are separated into four total daughter cells. The daughter cells are haploid and contain only one copy of each chromosome.
Reference
1. http://en.wikipedia.org/wiki/Chromosomal_crossover